Chirality: the property that gives molecules ‘hands’
Have you ever wondered why some people are right-handed and others are left-handed? Better yet, why can some people use both hands equally well? There are many articles out there trying to answer that question, and, from a human perspective, there are a few theories that give rise to our preferred handedness. Around 90% of people are predominantly right-handed, and this predominance appears to start in the brain. Language and communication control centers are generally governed in the left-hemisphere of the brain, which controls the right side of the body. However, for left-handed people, these centres are governed in the right-side of the brain [1]. From an evolutionary standpoint, it appears that use of a preferred hand was forced when we started standing on two legs instead of four [2]. However, in studies conducted with chimpanzees, they showed an equal preference of both left and right hands, with results showing 50% use of each [3].
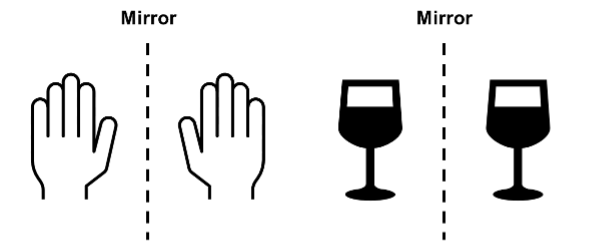
While this is all very interesting from a biological perspective, what if we told you that from a chemical perspective, molecules can also have this handedness. What’s more, when it comes to some molecules used in medicines, there is even a preferred molecular ‘hand’. Molecules that possess these ‘hands’ are called chiral molecules (coming from the Greek word ‘cheir’, which means hand). Any object can be termed chiral if its mirror image is non-superimposable on itself. For example, our own hands are chiral (our left hand is a mirror image of our right hand), yet a simple wine glass is achiral, i.e., not chiral.
CHIRAL ACHIRAL
At a molecular level, chirality in a molecule arises from an asymmetric carbon atom i.e., a carbon with 4 unique substituents bonded to it:
[4]
Molecules with this feature are found in abundance throughout nature. Amino acids that make up the proteins in our bodies are found in left-handed form, whereas simple sugars such as glucose, are most commonly found in right-handed form (however, a recent article in Chemistry World highlights that, although not so abundant in nature, opposite enantiomers, the term used to describe these mirror image forms, can be just as important in biological systems [5]).
L-Valine (amino acid)
D-Glucose (sugar)
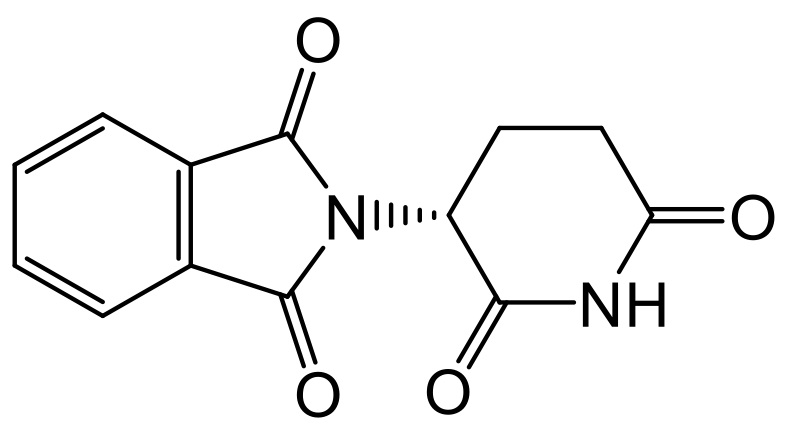
Different enantiomers can differ in tastes and smells, many of which we are familiar with, due to different biochemical reactions in the body. For example, L-asparagine (from asparagus) tastes bitter, whereas D-asparagine tastes sweet. This difference in biochemical reactions between enantiomers is extremely important to consider when synthesizing medicines. The importance of this was discovered in the 1960s when pregnant women who were given the drug Thalidomide (prescribed by doctors as a sedative) gave birth to babies with a wide variety of birth defects e.g. deformed limbs. It was soon discovered that while one enantiomer of thalidomide had strong sedative properties (R-enantiomer), the other one was high teratogenic i.e., causing these deformities (S-enantiomer).
(R)-Thalidomide
(S)-Thalidomide
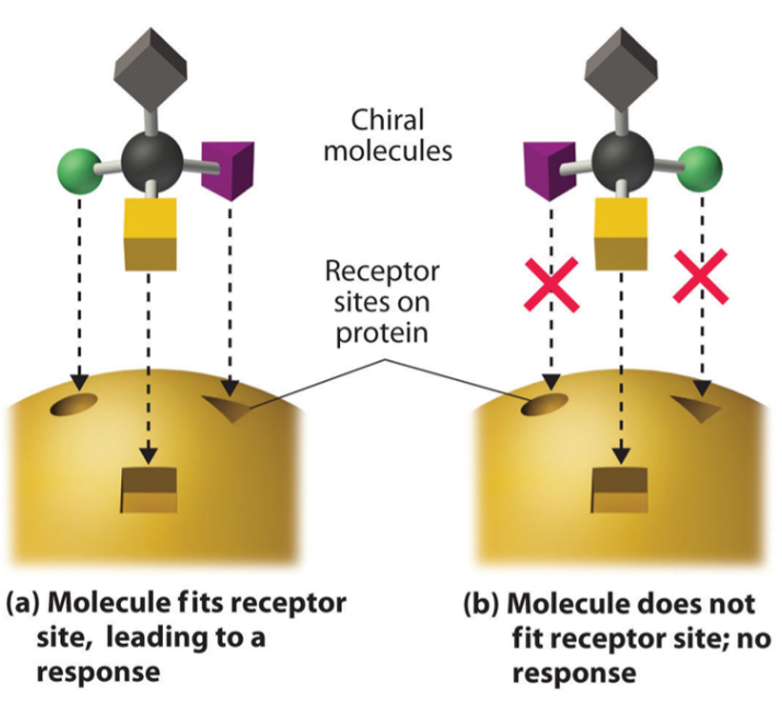
These types of biological reactions differ because receptors in the body tend to bind to only one enantiomer i.e., the medicine has a preferred ‘hand’ in generating a therapeutic response in the body. This means that the other chiral form of the drug does not bind with its intended receptor site, meaning that it has no effect on the body at all, or, it binds with another receptor site elsewhere in the body. If the latter occurs, either this other receptor site causes no harmful effects, or, a harmful or toxic effect can occur (such is the case with thalidomide).
[4]
Similar issues can also be found in other chiral medicines; Ibuprofen, an anti-inflammatory and commonly used painkiller, has one enantiomer which gives the desired anti-inflammatory response (the S- enantiomer), but the opposite enantiomer (R-enantiomer) is completely inactive (however, a portion of this inactive form is metabolically converted to its active form [6]). For naproxen, its S-enantiomer is used to treat patients with arthritis, but if they were given the R-enantiomer as treatment, they would end up with liver poisoning. Similarly with Ethambutol, its S-enantiomer is used to treat tuberculosis, but its R-enantiomer causes blindness.
(R)-Ibuprofen - Inactive
(R)-Naproxen - Causes liver poisoning
(R,R)-Ethambutol - Causes blindness
(S)-Ibuprofen - Anti-inflammatory
(S)-Naproxen - Treatment for arthritis
(S,S)-Ethambutol - Treatment for tuberculosis
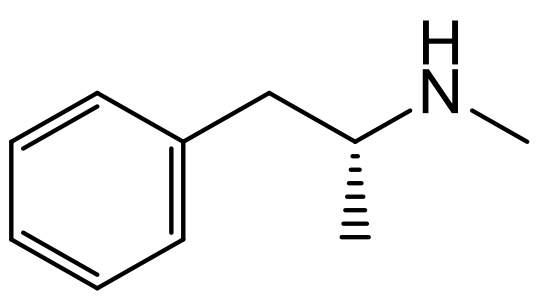
A particularly interesting one is methamphetamine, mostly due to its ‘popularity’ from the hit series Breaking Bad. We all tend to associate methamphetamine with being a purely illegal substance, which it is… but only D-methamphetamine. Its enantiomer, L-methamphetamine, is actually used in nasal sprays as a decongestant, which of course is completely legal!
D-Methamphetamine Illegal Substance
L-Methamphetamine Decongestant
It is clear that chiral molecules are incredibly important, and there is a lot of research going on around the world to further understand them, as well as to try and make their separation and purification faster and more efficient. This is has been the focus of my research for the last 8 years, and if you want to know more about the kind of work being done on chirality, you can read further on references [9]-[11].
References
[1] https://www.sciencefocus.com/the-human-body/why-are-some-people-left-handed/
[2] https://www.bbc.com/future/article/20141215-why-are-most-of-us-right-handed
[3] https://www.bbc.com/future/article/20160930-the-mystery-of-why-left-handers-are-so-much-rarer
[4] Coloured chiral molecules and receptor image - Stoker, H. S. General, Organic and Biological Chemistry, Sixth.; White, A., Ed.; Brooks/Cole, 2013.
[5] https://www.chemistryworld.com/features/lifes-chemistry-goes-through-the-looking-glass/4015507.article
[6] https://pubmed.ncbi.nlm.nih.gov/2207301/
[7] Breaking Bad Image - https://www.yalescientific.org/2013/12/the-chemistry-behind-breaking-bad/
[8] Nasal spray image - https://www.healthline.com/health/general-use/how-to-use-nasal-spray
[9] Dunn et al., 2019, Organic Process Research & Development, 23(9), 2031–2041. https://doi.org/10.1021/acs.oprd.9b00275
[10] Dunn et al., 2020, Crystal Growth and Design, 20(12), 7726–7741. https://doi.org/10.1021/acs.cgd.0c00974
[11] Nguyen et al., 2006, International Journal of Biomedical Science : IJBS, 2(2), 85–100. http://www.ncbi.nlm.nih.gov/pubmed/23674971